
slider-informatie-nefrotisch-syndroom

The aim of the REducing STEroids in Relapsing Nephrotic syndrome (RESTERN) study is to show that at least equal clinical benefits can be obtained by reduced corticosteroid exposure, which minimizes toxicity in children with nephrotic syndrome. In order to achieve this aim, the RESTERN study will address the following objectives:
1. Conducting a nation-wide randomized placebo-controlled trial aiming to reduce steroid exposure in nephrotic syndrome relapses;
2. Starting a nephrotic syndrome registry, in which data can be added by patients and professionals;
3. Characterizing patients with nephrotic syndrome and creating a biobank for studies on the pathogenesis of nephrotic syndrome in children and the identification of biomarkers that predict diagnosis, prognosis and treatment efficacy.
* Levamisole, ciclosporine, tacrolimus, mycofenolaat mofetil (Cellcept®), mycofenolaat sodium (Myfortic®), prednisone max. 4mg/m2 on alternate days, Cyclophosphamide, rituximab
** defined as Albustix positive proteinuria (3+ or greater) for three consecutive days or the presence of generalised edema plus 3+ proteinuria
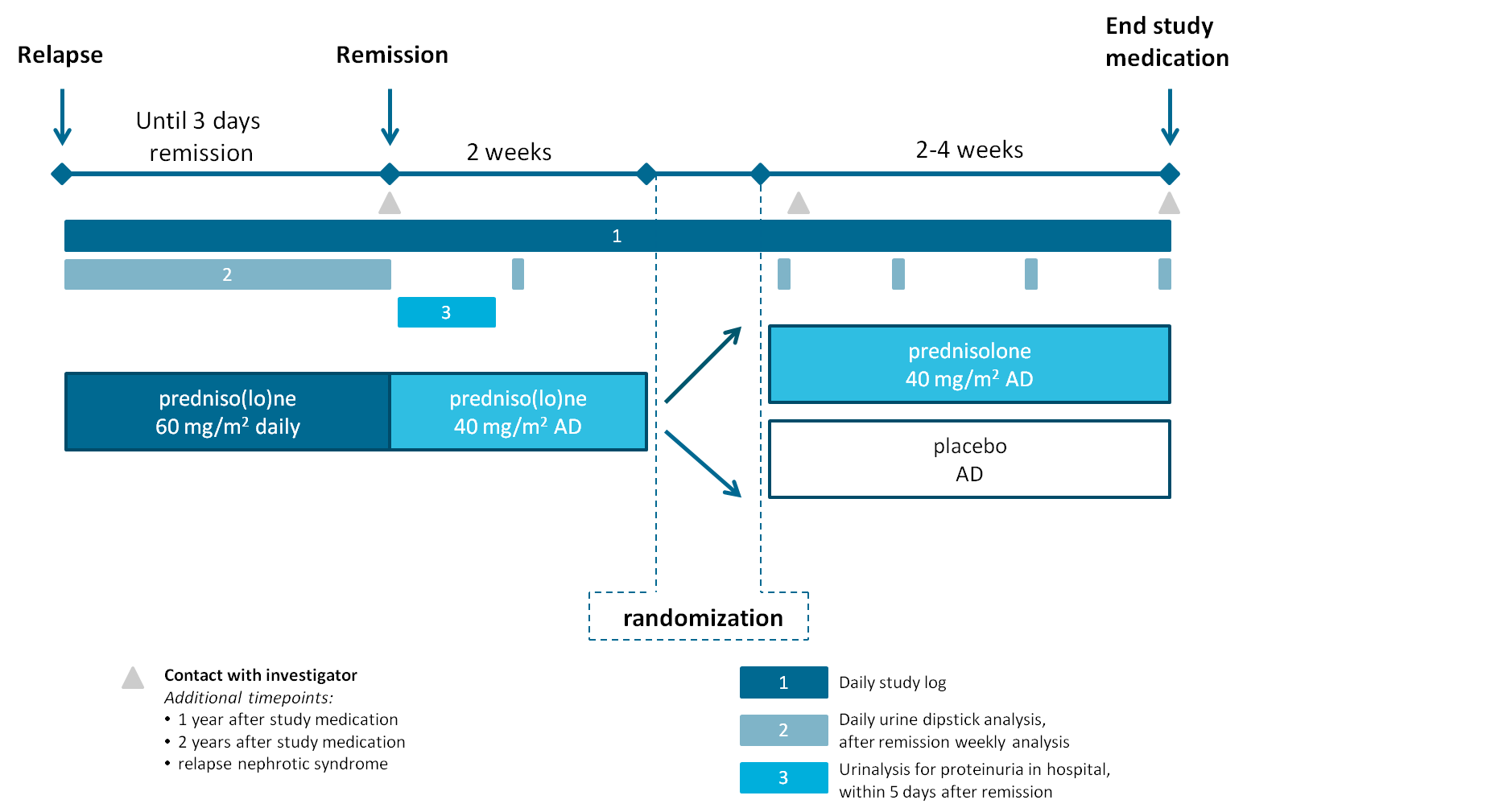
Primary outcome
Secondary outcome
If you have any questions, comments or suggestions, please do not hesitate to contact us using the contact form or restern.kg@radboudumc.nl
If you have a question or comment, we’d love to hear it. For this you can fill in the contact form and we will contact you as soon as possible. For referring patients we would like to ask you to contact us through the referrer phone number.
